Company told to stop supplying and selling losartan products
KUALA LUMPUR, Nov 24 (Bernama) -- A company that supplies and sells products containing losartan has been ordered to stop doing so, says Health director-general Datuk Noor Hisham Abdullah.
He said in a statement that the order would be effective until the company could prove that its products do not contain impurity N-nitrosodiethylamine (NDEA).
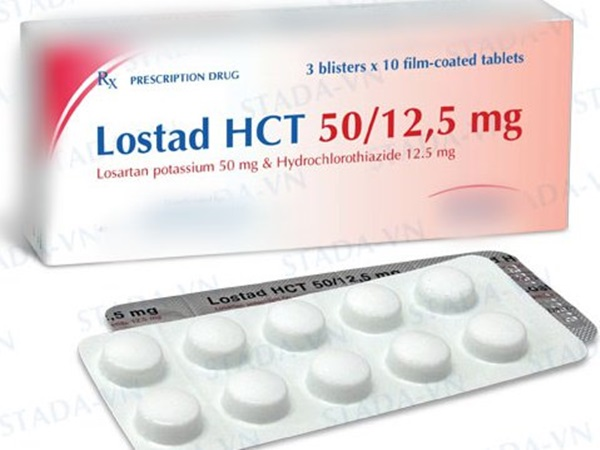
“There’s only one losartan product in Malaysia and that’s Lostad HCT 50/12.5 mg film-coated tablets. Products containing NDEA may cause cancer to consumers in the long run.
“Patients taking this medicine are advised not to abruptly stop taking it and to seek advice from medical professionals,” he said, adding that the public should use products registered with the Drug Control Authorities.
Products containing losartan and valsartan are used to treat patients with high blood pressure.
The statement was issued following a recall notice by the United States Food and Drug Administration (USFDA) of products containing active valsartan and losartan ingredients due to the presence of NDEA.
Dr Noor Hisham said the public can check the registration status of products via the National Pharmacy Regulatory Agency website at www.npra.gov.my or through the NPRA Product Status app which would be downloaded from Google Play.
-- BERNAMA
HealthEdge
EXCLUSIVE

Pet Vaccination, Public Awareness And Surveillance Key Towards Rabies-free Southeast Asia - Experts
KUCHING, Dec 11 (Bernama) -- The goal of making Southeast Asia free from human rabies can be achieved through a total understanding of the disease, how it can be prevented and responsible pet ownership among communities, say experts.
read more ››IN FOCUS

TAVI KAEDAH BAIK PULIH INJAP JANTUNG TANPA PEMBEDAHAN