Vivantis Technologies Elevates COVID-19 Testing Capacity In Malaysia

By Nadia Jumri
KUALA LUMPUR (Bernama) – The world has been battling COVID-19 for close to a year now, with more than 200 countries and territories affected by the pandemic.
In Malaysia, the third wave of infections led to total case numbers spiking to over 75,000. Globally, the virus has infected more than 68 million people and caused over 1.5 million fatalities.
Due to the virus’ highly virulent nature, producing COVID-19 test kits has become a top priority for certain biotechnology companies focusing on research and development (R&D) in Malaysia.
Among them is Vivantis Technologies Sdn Bhd (Vivantis Technologies) which, in collaboration with Bioeconomy Corporation, is expanding its production capacity to become the emerging leading manufacturer of COVID-19 test kits in Southeast Asia.
Incorporated in 2002, Vivantis Technologies is a homegrown research-based biotechnology company that has been awarded BioNexus status by Bioeconomy Corporation for providing integrated solutions for the life-science industry.
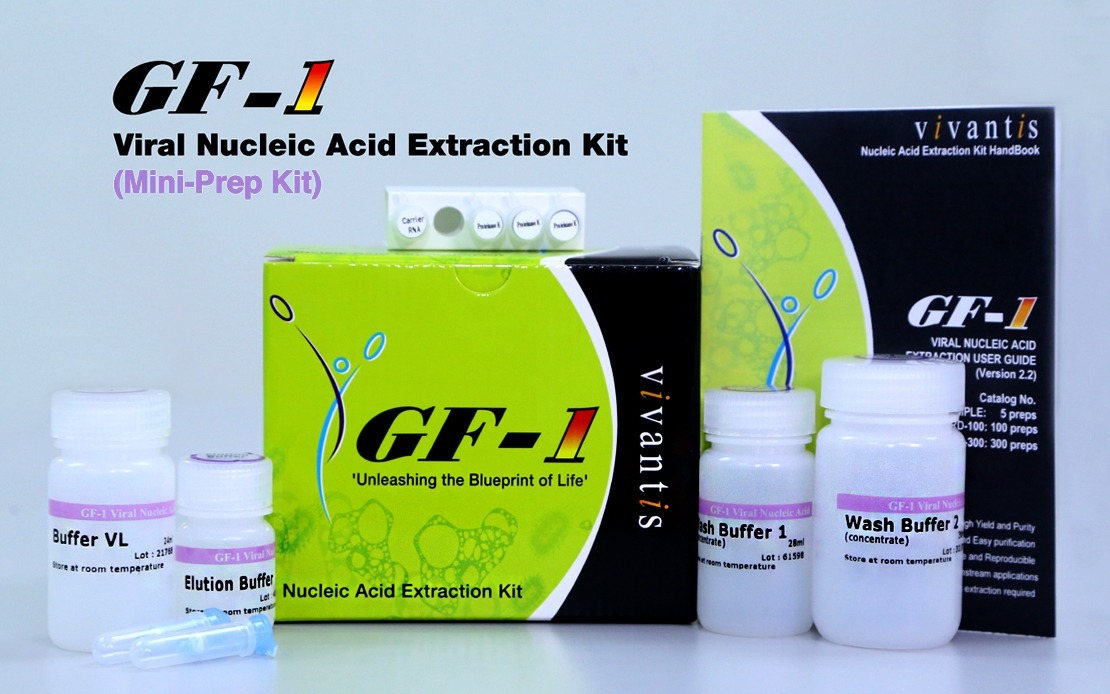
The GF-1 Viral Nucleic Acid Extraction Kit efficiently isolates and purify viral DNA and/or RNA from a broad range of viral samples, including VTM buffers consisting of COVID-19 virus-infected swabs.
Vivantis Technologies specialises in the production of restriction enzymes, deoxyribonucleic acid (DNA) extraction kits, DNA amplification reagents and other related products for molecular biology research.
RT-PCR TEST KIT PROTOTYPE
pic-3
Vivantis Technologies founder and chief executive officer Law Eng Lim said the company, which has a portfolio of start-up ventures, recently developed two prototypes of locally-made polymerase chain reaction (RT-PCR) COVID-19 test kits, namely Vivantis GF-1 Viral Nucleic Acid Extraction Kit (Mini Prep Kit) and Vivantis Viprime Plus 2019-nCOV Multiplex RT-qPCR Kit (Master Mix).
The Mini Prep Kit allows rapid and efficient purification of viral DNA or ribonucleic acid (RNA) from samples such as serum, plasma, body fluid, virus-infected cell culture supernatant and viral transport media buffers consisting of virus-infected swabs.
The Master Mix is a ready-to-use solution with all necessary components (primer, probe and master mix) in a single mix.
“This (Master Mix) is an innovative handheld portable device that is fast, simple (to use) and cheap,” Law told Bernama in an interview here recently.
He said the Master Mix test kit has 99.9 percent sensitivity and specificity and has been validated by local and international laboratories and universities, including Universiti Malaysia Sarawak and the Medical Molecular Biology Institute at Universiti Kebangsaan Malaysia.
“We also have done the validation (for our test kits) in spin-off laboratories from the Institute of Pasteur in France and also in some countries where we have dealers for our products.
“Distributors who wish to sell our products must first get them validated by their respective countries. After the testing is done, they would send us the validation results,” he said.
VIVANTIS TECHNOLOGIES – THE UNSUNG HERO OF COVID-19 FRONTLINE WORKERS
pic-4
Law said after the COVID-19 outbreak was first detected in Malaysia, Vivantis Technologies, which operates through three laboratories in Selangor, had to ramp up their production in order to help the Ministry of Health (MOH) with its testing efforts.

Law Eng Lim.
“When Malaysia reported its first few COVID-19 cases (in January), our labs were stretched to their limit in producing a test kit that is designed based on the recommended protocols posted by the World Health Organisation.
“We really had to ramp up our production… actually, we were also part of the frontliners that people don’t see as we were also involved in the fight against COVID-19. My people were forced to work extra hours and rushed to produce the test kits,” he said.
The company is also working closely with the Medical Molecular Biology Institute which is conducting COVID-19 swab tests using Vivantis Technologies’ test kits.
Law, however, said the company is facing two major challenges in producing the COVID-19 test kits, namely procuring raw materials and manpower.
“We hope to work together with MOH so that our test kits can be supplied to every healthcare facility under the ministry,” he said.
SUPPLYING TO OTHER COUNTRIES
pic-2
With over 15 years of experience in the biotech sector, Vivantis Technologies has been capitalising on its technological know-how and best practices to produce the COVID-19 test kits.
Global demand for COVID-19 test kits is likely to surge in the coming days as the number of new cases rises steeply worldwide.
According to Law, Vivantis Technolgies is now prepared to supply the kits to Southeast Asian countries, as well as India.
“We are a small company and we want to be able to sell our test kits to other countries such as the Philippines, Indonesia and other developing countries. With that, Malaysia can play a role as a test kit supplier,” he added.
To meet the demand for its test kits, the company had to increase its production capacity to make up to five to seven million kits a month, thus turning Vivantis Technologies into one of the largest COVID-19 test kit manufacturers in Southeast Asia.
Law also revealed that his company has submitted an application to the United States Food and Drug Administration seeking its approval to distribute and market their test kits in that country.
Vivantis Technologies has also received tremendous support from organisations such as Malaysia Productivity Corporation-Chemical Productivity Nexus (MPC-CPN), a government body that is committed to improving the productivity of the chemicals and chemical products subsectors.

Law routinely visits his laboratory to engage with the R&D team from Vivantis Technologies.
MPC-CPN has recognised the work done by Vivantis Technologies which can significantly influence other local enterprises to systematically address the barriers that prohibit them from moving towards high value-added segments.
Law said Bioeconomy Corporation also assisted Vivantis Technologies to seek the approval of the Ministry of International Trade and Industry to allow their operations to run during the Movement Control Order period earlier this year.
CHANGES IN COVID-19 TESTING IN THE FUTURE
pic-5
Law, meanwhile, said COVID-19 test developers all over the world are now racing to produce next-stage technologies that could allow rapid and widespread testing to be carried out.
“This is a multi-billion dollar business and everyone is racing to come out with the COVID-19 test kits,” he said.
In the near future, there may be changes in terms of the method of using the COVID-19 test kits, he said, adding that Vivantis Technologies’ R&D team is working hard to improve the test kits in two areas.
“Our dedicated R&D team recently developed an application note that suggests the use of this test kit in extracting nucleic acid from saliva samples.
“We also plan to develop a semi-automation system for extraction of the viral sample. This means, in the future, it need not be done manually but pretty much by a machine. This will reduce the risk of infection and increase the consistency of the testing performance,” he added.

Law and the R&D team from Vivantis Technologies presenting the performance data of ViPrimePLUS 2019-nCoV Multiplex RT-qPCR Kit to a potential customer.
Edited by Rema Nambiar
BERNAMA
For more information click here
HealthEdge
EXCLUSIVE

Pet Vaccination, Public Awareness And Surveillance Key Towards Rabies-free Southeast Asia - Experts
KUCHING, Dec 11 (Bernama) -- The goal of making Southeast Asia free from human rabies can be achieved through a total understanding of the disease, how it can be prevented and responsible pet ownership among communities, say experts.
read more ››IN FOCUS

TAVI KAEDAH BAIK PULIH INJAP JANTUNG TANPA PEMBEDAHAN



